Monthly Archives: July 2021
Results:
https://www.zenodo.org/search?page=1&size=20&q=ilbeigi
Since most of the plant hormones are not volatile and have high boiling points, in the first step a high temperature injection port system was designed and constructed to bring the samples into gas phase (Figure 1). The injection port operates in temperature range of 25-260o Performance the injection port was assessed for both vaporization and transporting the samples into the ionization region of the ion mobility spectrometry (IMS). It was successfully tested for measurements of the non-volatile plant hormones including auxin, salicylic acid.

Figure 1. Photos of the constructed high temperature injection port.
- In this work, an IMS-based method was developed to exploit the advantages of SMPE, MCC and IMS for fast and sensitive analysis of real samples in complex matrix. The SPME-MCC-IMS method was employed for quantitative analysis of MeSA in tomato leaves.
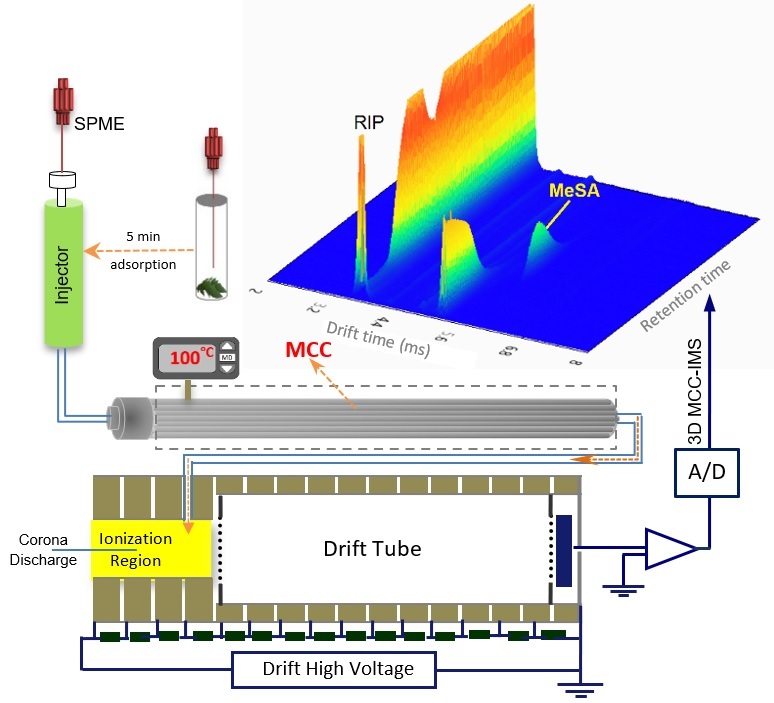
Figure 2. Schematic presentation of the experimental set-up (SPME-MCC-IMS).

Figure 3. Photo of coupling SPME-MCC-IMS.
-
Detection of auxin plant hormones by ion mobility-mass spectrometry
https://link.springer.com/article/10.1007/s00216-022-04198-x
https://www.zenodo.org/record/7981083
Ion mobility spectrometry (IMS) equipped with a corona discharge (CD) ion source was used for measurement of three auxin plant hormones including indole-3-acetic acid (IAA), indole-3-propionic acid (IPA), and indole-3-butyric acid (IBA). The measurements were performed in both positive and negative polarities of the CD ion source. Dopant gases NH3, CCl4, and CHBr3were used to modify the ionization mechanism. A time-of-flight mass spectrometer (TOFMS) orthogonal to the IMS cell was used for identification of the product ions. Density functional theory was used to rationalize formation of the ions, theoretically. The mixtures of the auxins were analysed by CD-IMS. The separation performance depended on the ion polarity and the dopants. In the positive polarity without dopants, auxins were ionized via protonation and three distinguished peaks were observed.Application of NH3dopant resulted in two ionization channels,protonation and NH4+ attachment leading to peak overlapping. In the negative polarity two ionization reactions were operative, via deprotonation and O2– attachment.The separation of the monomer peaks was not achieved while the peaks of anionic dimers [2M-H]– were separated well. The best LOD (4 ng) was obtained in negative polarity with CCl4dopant.Methylation (esterification) of IAA improved LODs by about one order.
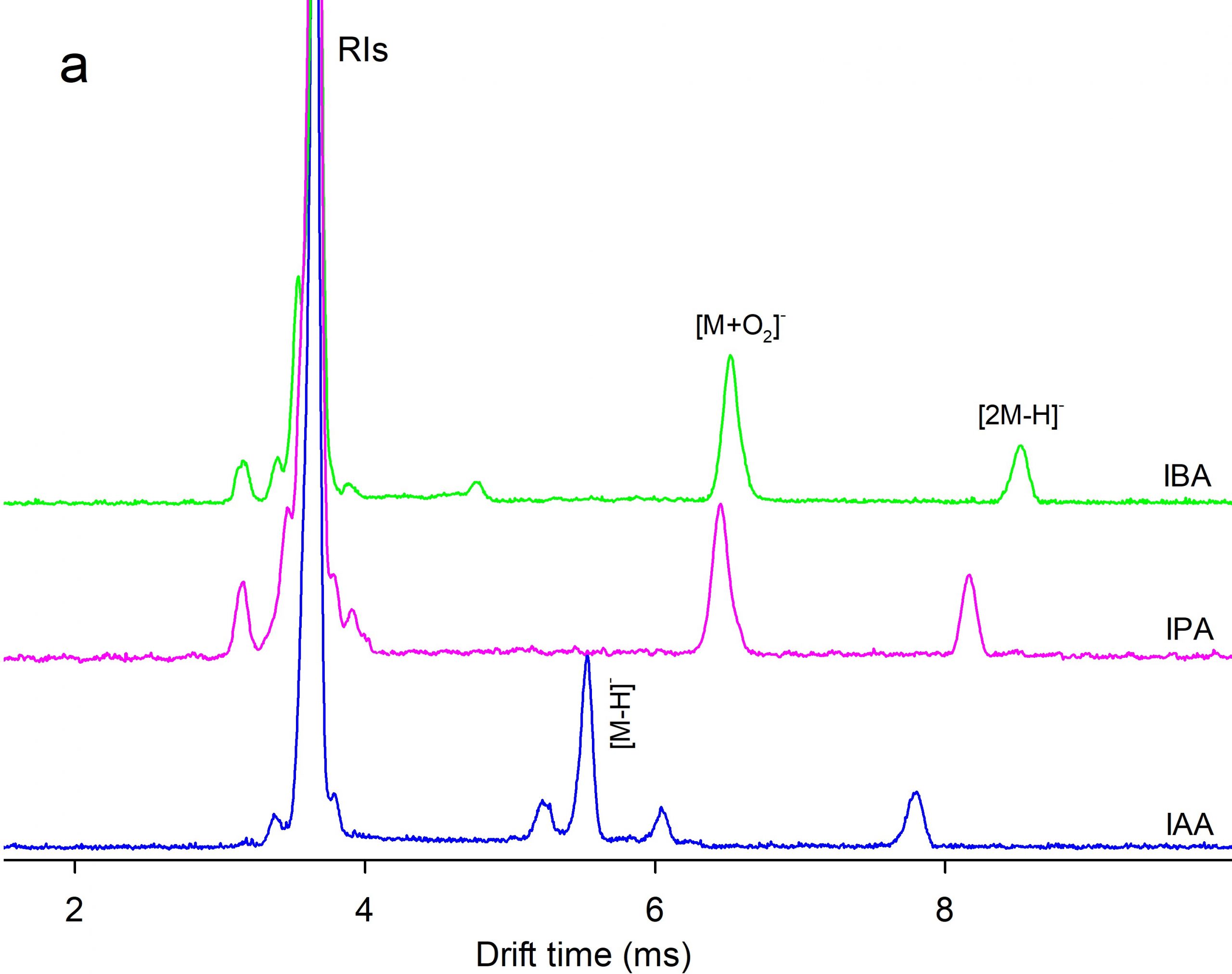
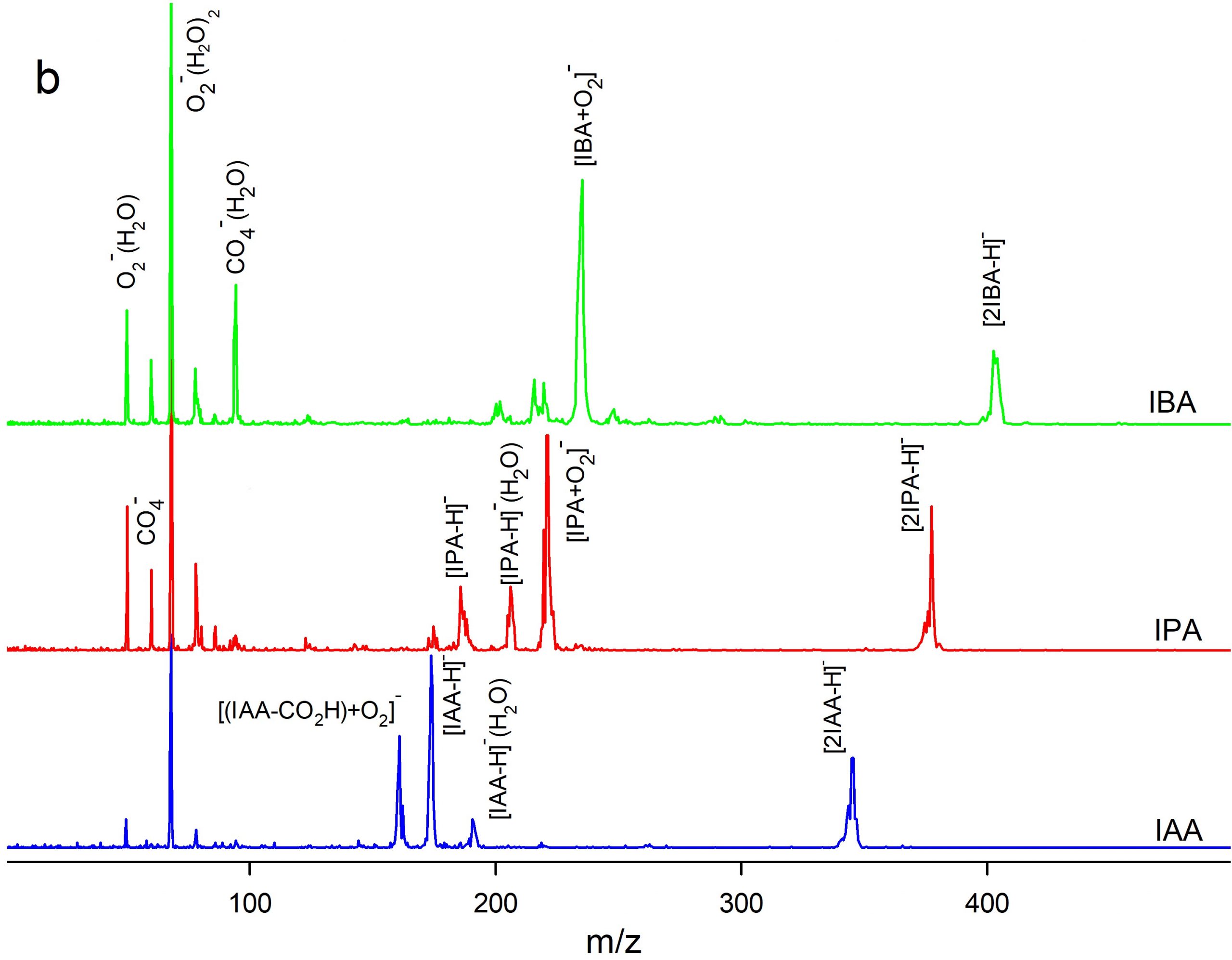
Figure 4. Comparison of (a) ion mobility and (b) mass spectra of IAA, IPA, and IBA in the negative mode of CD ion source.
-
Detection of Methyl Salicylate in Tomato Leaves
https://pubs.acs.org/doi/pdf/10.1021/acs.jafc.2c05570
https://www.zenodo.org/record/7981306
Methyl salicylate (MeSA) is a plant-signaling molecule that plays an essential role in the regulation of the plant responses to biotic and abiotic pathogens. In this work, solid phase microextraction (SPME) and multi-capillary column (MCC) are coupled to ion mobility spectrometer (IMS) to detect MeSA in tomato leaves. The SPME-MCC-IMS method provides two-dimensional (2D) separation by both MCC and IMS, based on the retention and drift times. The effect of the IMS polarity on the separation efficiency of MCCs was also investigated. In the positive polarity, ionization of MeSA resulted in ([MeSA+H]+) while in the negative deprotonated ions ([MeSA-H]–) and O2– adduct ion ([MeSA+O2]– were formed. In the real sample analysis, the negative polarity operation resulted in the suppression of many matrix molecules and thus in the reduction of interferences. Four different SPME fibers were used for head space analysis and four MCC columns were investigated. In the negative polarity, complete separation was achieved for all the MCCs columns. The limits of detection (LODs) of 15 and 22 ppb (v/v) were achieved for the direct injection of head space of MeSA in positive and negative polarities, indicating high sensitivity of IMS toward MeSA. Limits of detection (LOD) of 0.1 µg g-1 and linear range of 0.25-14 µg g-1 were obtained for measurement of MeSA by the SPME-MCC-IMS method with 5 min extraction time. The MeSA content of fresh tomato leaves were determined as 1.5-9.8 µg g-1, 24-96 h after inoculation by tomato mosaic virus (ToRSV).
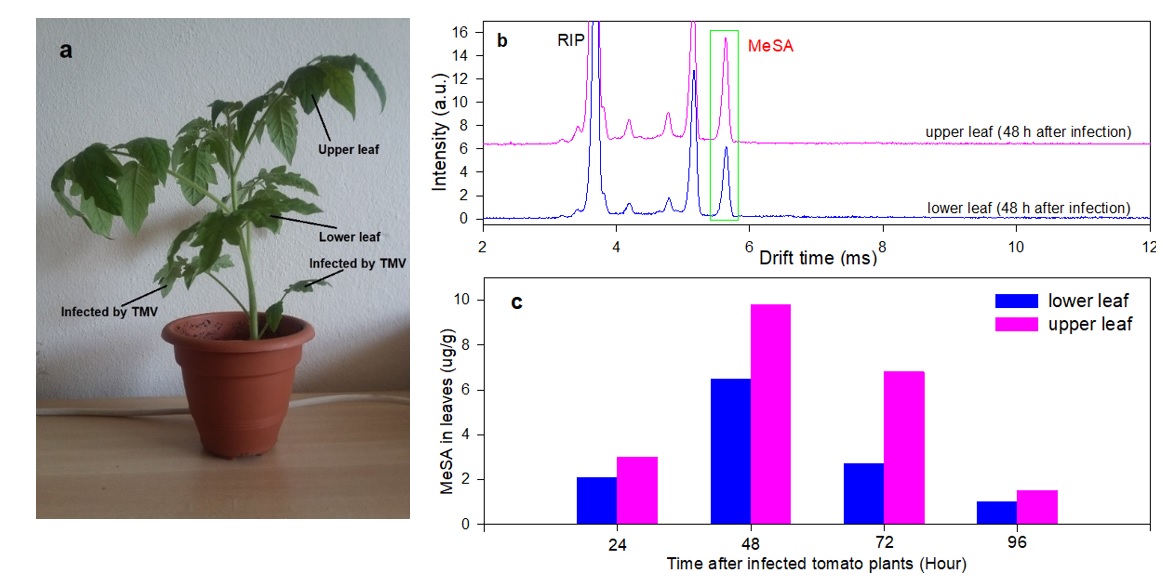
Figure 5. (a) The ToRSV inoculated, lower and upper leaves in a typical tomato plant. (b) The MCC-separated IMS spectra obtained 48 h after inoculation by ToRSV. (c) The measured MeSA content of upper and lower leaves 24 to 96 hours after inoculation.
The following link shows inoculation of the lower leaves of the tomato plants with an age of 5 weeks by tomato ringspot virus (ToRSV)

https://www.zenodo.org/record/7981460
-
Introduction of formic acid as a dopant for analysis of plant hormones
In this work, we introduce formic acid (FA) as a potent dopant for APCI ionization in the negative polarity, which can be applied to IMS and MS techniques. To assess the efficiency of this dopant, ionization of several analytes including plant hormones, drugs, explosives, and pesticides was investigated with and without FA dopant. This work is under review now.
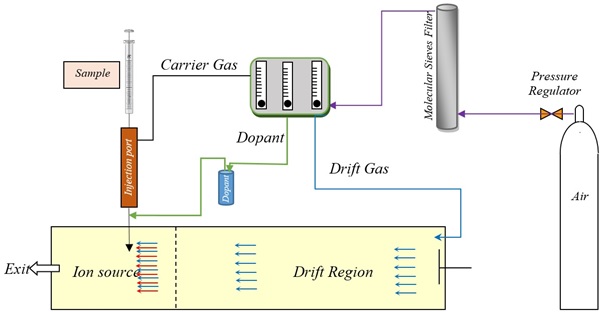
Figure S1. Schematic representation of the experimental setup and the gas flow paths.
-
Measurement of cytokinins in coconut juice by LC-IM-QTOF
Cytokinins play various important functions in cell division, chlorophyll formation, stimulating differentia tion of plant tissues, seed germination, bud formation, release of buds from apical dominance, leaf expansion, and reproductive development.
Cytokinins are in very low concentrations in plants. To overcome this limitation, solid-phase extraction (SPE) is used as a pre-concentration and purification method.
In this project, three cytokinins including zeatin, kinetin, 6-Benzylaminopurine (BAP) were measured in coconut juice in collaboration with BOKU university in Vienna. An Agilent 6560 LC-IM-QTOF mass spectrometer was used for all measurements. A Dual Jetstream electrospray ion source (ESI) was used for ionisation of the compounds.
-
In-site measurement of methyl salicylate in tomato
Plants release some volatiles compounds and plant hormones when they are exposed to different pathogens and mechanical damages. In this project, the released plant hormones from blubbery and tomato were studied and analyzed. To monitor the released plant hormones, a device with different components for collecting the chemicals was designed and constructed. The designed device includes a Teflon base with a hole in center to put the plant into it (Figure 8a and b) and a glass cylinder (2L) which is sealed around the plant to avoid leakage of the emitted chemicals (Figure 8c). A stream of zero air with flow rate of 700 ml was used to sweep up the emitted volatiles and pass them through the outlet in the top of the glass cylinder. The outlet of the glass cylinder was equipped with a Tenax adsorbent (Figure 8d) to collected the exited volatile compounds.

Figure 8. Experimental setup for collecting the emitted volatile chemicals and plant hormones from tomato plant including (a) a Teflon guillotine-like base, (b) an aluminum stool, (c) glass cylinder and pump, and (d) the Tenax trap as an adsorbent.
-
In-site analysis of VOCs emitted from blueberry leaves
Herbivore-induced plant volatiles (HIPVs) are emitted from plants after they are attacked by herbivores . The production and release of the HIPVs are mainly controlled by the defensive plant hormone jasmonic acid (JA) and its ester derivative methyl jasmonate (MeJA). Ocimene, benzene acetonitrile, and caryophyllene are the three main HIPVs emitted by plants. In this part, we study the emitted HIPVs from blueberry leaves in three conditions: (i) after mechanical punching of the blueberry leaves; (ii) after spraying MeJA on the leaves; and (iii) after herbivore attack.

The experimental set-up for collection volatiles from blueberry plant.

The infested blueberry plant by insect.